X-ray Diffraction
X-ray scattering techniques are powerful and nondestructive methods for examining the atomic structural and physical characteristics of crystalline and noncrystalline materials. The most commonly used scattering method is X-ray diffraction (XRD). Powder XRD provides information on crystal structure, phase identity, preferred crystal orientation (texture), and other structural parameters, such as average grain/crystallite size, lattie strain, and crystal defects. The peaks in a powder XRD pattern are produced by constructive interference of a monochromatic beam of X-rays diffracted at specific angles from each set of lattice planes in a sample. The peak intensities are determined by the distribution of atoms within the lattice. Consequently, the X-ray diffraction pattern is the fingerprint of the periodic atomic arrangements in a given material. Comparing a sample’s XRD pattern to a database of known structures enables the phase identification of a large variety of crystalline and nanocrystalline samples. This method is quantitative and requires minimal or no sample preparation. Other X-ray scattering methods include small-angle X-ray scattering (SAXS), wide-angle X-ray scattering (WAXS), grazing-incidence XRD, X-ray reflectivity, and total scattering for pair distribution function (PDF) analysis.
APPLICATIONS
- Natural and synthetic samples as powders, pressed pellets, single- and multi-layer thin films, and more*
- Identification/quantification of crystalline and amorphous phases
- Measurement of average crystallite size, strain, or micro-strain effects
- Determination of the ratio or percentage of crystalline to amorphous material in bulk materials and thin-films
- Detecting minor crystalline phases (at concentrations greater than ~1%)
- Measuring sub-milligram loose powder or dried solution samples for phase identification
- Measurement at ambient conditions
*Other sample types may be possible - inquire with questions.
LIMITATIONS
- Identification of amorphous solids (requires suitable references/standards)
- Wet samples/suspensions (except high solid:liquid ratio)
Panalytical Empyrean Multipurpose Diffractometer
The Empyrean Nano Edition is a multipurpose X-ray diffraction platform equipped to perform a wide range of x-ray scattering measurements including:
- High-resolution powder x-ray diffraction (pXRD)
- Small-angle x-ray scattering (SAXS) and wide-angle x-ray scattering (WAXS)
- X-ray reflectivity (XRR)
- Grazing-incidence x-ray diffraction (GI-XRD)
- Total scattering for pair distribution function (PDF) analysis
Our system is also equipped with following a sample changer for pXRD, a vacuum chamber for SAXS/WAXS, a silver (Ag) x-ray source and capillary spinner for PDF, and a motorized X,Y,Z stage for XRR and GI-XRD. We also have incident and diffracted optics for each type of experiment.
TECHNICAL SPECIFICATIONS
- Signal Detected: Diffraction and scattering (elastic)
- Detectors Available
- Galipix3D area detector with CdTe sensor (suitable for Cu and Ag radiation)
- Proportional detector (Cu radiation only)
- Anode Types Available (sealed high-resolution x-ray tubes)
- Copper (Kalpha1 = 1.5406 A)
- Silver (Kalpha1 = 0.5595 A)
- All elements, assuming they are present in a crystalline matrix (lightest elements e.g., hydrogen likely not detectable)
- Detection Limits: Quantitative multiphase analysis: ~1%
- Minimum film thickness for phase identification: ~2 nm
University of Akron (Nita Sahai, PI)
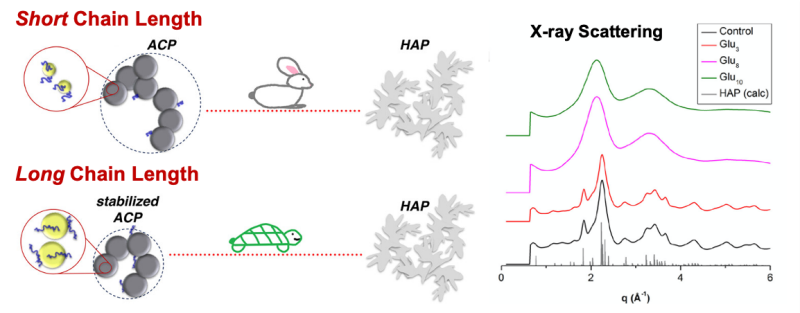
Effects of Biomacromolecules on Calcium Phosphate Formation and Transformation: Calcium phosphates are the main inorganic mineral component of bones and teeth and are of interest in fields ranging from bioengineering to Earth science. Biomineralization of calcium phosphates is complex due to the effects of biomacromolecules such as proteins. This research examined the effects of model organic molecules on the formation of and transformation of calcium phosphates. The experiments involved synthesizing amorphous calcium phosphate in the presence of different length peptides and observing its transformation to crystalline hydroxylapatite. The experiments used a suite of complementary characterization methods including two x-ray scattering techniques made possible through collaboration with NanoEarth scientists. The results of this two-part investigation showed how organic additives influence the size of the amorphous calcium phosphate and the kinetics of its crystallization. Based on these results the study also proposed a specific mechanism to explain the effect of organic molecule chain length. These findings are an important step towards understanding how biological systems can control mineralization processes and will improve the design of biomimetic mineralized materials for biomedical applications.
Publications:
- Ustriyana, P., Harmon, E., Chen, K., Michel, F. M., & Sahai, N. (2020) Oligo (L-Glutamic Acids) in Calcium Phosphate Precipitation: Chain length effect. The Journal of Physical Chemistry B, 124, 6278-6287, https://doi.org/10.1021/acs.jpcd.0c01689.
- Ustriyana, P., Michel, F. M., Wilson, M. C., Harmon, E., Chen, J. & Sahai, N. (2020) Oligo (LGlutamic Acids) in Calcium Phosphate Precipitation: Mechanism of delayed transformation. The Journal of Physical Chemistry B, 124, 6288-6298, https://doi.org/10.1021/acs.jpcd.0c01690.
Figure 1. Left: Conceptual model showing the effects of short- vs. long-chain organic molecules on the size and rate of calcium phosphate formation and transformation. Right: Example of x-ray scattering data used to study the effects of the different organic molecules.
- Ustriyana, P., Harmon, E., Chen, K., Michel, F. M., & Sahai, N. (2020) Oligo (L-Glutamic Acids) in Calcium Phosphate Precipitation: Chain length effect. The Journal of Physical Chemistry B, 124, 6278-6287, https://doi.org/10.1021/acs.jpcd.0c01689.
- Ustriyana, P., Michel, F. M., Wilson, M. C., Harmon, E., Chen, J. & Sahai, N. (2020) Oligo (LGlutamic Acids) in Calcium Phosphate Precipitation: Mechanism of delayed transformation. The Journal of Physical Chemistry B, 124, 6288-6298, https://doi.org/10.1021/acs.jpcd.0c01690.
- Rod, K. A., Smith, A. P., Leng, W., Colby, S., Kukkadapu, R. K., Bowden, M., Qafoku, O., Um, W., Hochella, M. F., Jr., Bailey, V. L. and Renslow, R. S. (2020). Water-dispersible nanocolloids and higher temperatures promote the release of carbon from riparian soil.. Vadose Zone Journal, 19(1), e20077. https://doi.org/10.1002/vzj2.20077
Coming soon...
X-ray scattering techniques are powerful and nondestructive methods for examining the atomic structural and physical characteristics of crystalline and noncrystalline materials. The most commonly used scattering method is X-ray diffraction (XRD). Powder XRD provides information on crystal structure, phase identity, preferred crystal orientation (texture), and other structural parameters, such as average grain/crystallite size, lattie strain, and crystal defects. The peaks in a powder XRD pattern are produced by constructive interference of a monochromatic beam of X-rays diffracted at specific angles from each set of lattice planes in a sample. The peak intensities are determined by the distribution of atoms within the lattice. Consequently, the X-ray diffraction pattern is the fingerprint of the periodic atomic arrangements in a given material. Comparing a sample’s XRD pattern to a database of known structures enables the phase identification of a large variety of crystalline and nanocrystalline samples. This method is quantitative and requires minimal or no sample preparation. Other X-ray scattering methods include small-angle X-ray scattering (SAXS), wide-angle X-ray scattering (WAXS), grazing-incidence XRD, X-ray reflectivity, and total scattering for pair distribution function (PDF) analysis.
APPLICATIONS
- Natural and synthetic samples as powders, pressed pellets, single- and multi-layer thin films, and more*
- Identification/quantification of crystalline and amorphous phases
- Measurement of average crystallite size, strain, or micro-strain effects
- Determination of the ratio or percentage of crystalline to amorphous material in bulk materials and thin-films
- Detecting minor crystalline phases (at concentrations greater than ~1%)
- Measuring sub-milligram loose powder or dried solution samples for phase identification
- Measurement at ambient conditions
*Other sample types may be possible - inquire with questions.
LIMITATIONS
- Identification of amorphous solids (requires suitable references/standards)
- Wet samples/suspensions (except high solid:liquid ratio)
Panalytical Empyrean Multipurpose Diffractometer
The Empyrean Nano Edition is a multipurpose X-ray diffraction platform equipped to perform a wide range of x-ray scattering measurements including:
- High-resolution powder x-ray diffraction (pXRD)
- Small-angle x-ray scattering (SAXS) and wide-angle x-ray scattering (WAXS)
- X-ray reflectivity (XRR)
- Grazing-incidence x-ray diffraction (GI-XRD)
- Total scattering for pair distribution function (PDF) analysis
Our system is also equipped with following a sample changer for pXRD, a vacuum chamber for SAXS/WAXS, a silver (Ag) x-ray source and capillary spinner for PDF, and a motorized X,Y,Z stage for XRR and GI-XRD. We also have incident and diffracted optics for each type of experiment.
TECHNICAL SPECIFICATIONS
- Signal Detected: Diffraction and scattering (elastic)
- Detectors Available
- Galipix3D area detector with CdTe sensor (suitable for Cu and Ag radiation)
- Proportional detector (Cu radiation only)
- Anode Types Available (sealed high-resolution x-ray tubes)
- Copper (Kalpha1 = 1.5406 A)
- Silver (Kalpha1 = 0.5595 A)
- All elements, assuming they are present in a crystalline matrix (lightest elements e.g., hydrogen likely not detectable)
- Detection Limits: Quantitative multiphase analysis: ~1%
- Minimum film thickness for phase identification: ~2 nm
University of Akron (Nita Sahai, PI)
Effects of Biomacromolecules on Calcium Phosphate Formation and Transformation: Calcium phosphates are the main inorganic mineral component of bones and teeth and are of interest in fields ranging from bioengineering to Earth science. Biomineralization of calcium phosphates is complex due to the effects of biomacromolecules such as proteins. This research examined the effects of model organic molecules on the formation of and transformation of calcium phosphates. The experiments involved synthesizing amorphous calcium phosphate in the presence of different length peptides and observing its transformation to crystalline hydroxylapatite. The experiments used a suite of complementary characterization methods including two x-ray scattering techniques made possible through collaboration with NanoEarth scientists. The results of this two-part investigation showed how organic additives influence the size of the amorphous calcium phosphate and the kinetics of its crystallization. Based on these results the study also proposed a specific mechanism to explain the effect of organic molecule chain length. These findings are an important step towards understanding how biological systems can control mineralization processes and will improve the design of biomimetic mineralized materials for biomedical applications.
Publications:
- Ustriyana, P., Harmon, E., Chen, K., Michel, F. M., & Sahai, N. (2020) Oligo (L-Glutamic Acids) in Calcium Phosphate Precipitation: Chain length effect. The Journal of Physical Chemistry B, 124, 6278-6287, https://doi.org/10.1021/acs.jpcd.0c01689.
- Ustriyana, P., Michel, F. M., Wilson, M. C., Harmon, E., Chen, J. & Sahai, N. (2020) Oligo (LGlutamic Acids) in Calcium Phosphate Precipitation: Mechanism of delayed transformation. The Journal of Physical Chemistry B, 124, 6288-6298, https://doi.org/10.1021/acs.jpcd.0c01690.
Figure 1. Left: Conceptual model showing the effects of short- vs. long-chain organic molecules on the size and rate of calcium phosphate formation and transformation. Right: Example of x-ray scattering data used to study the effects of the different organic molecules.

- Ustriyana, P., Harmon, E., Chen, K., Michel, F. M., & Sahai, N. (2020) Oligo (L-Glutamic Acids) in Calcium Phosphate Precipitation: Chain length effect. The Journal of Physical Chemistry B, 124, 6278-6287, https://doi.org/10.1021/acs.jpcd.0c01689.
- Ustriyana, P., Michel, F. M., Wilson, M. C., Harmon, E., Chen, J. & Sahai, N. (2020) Oligo (LGlutamic Acids) in Calcium Phosphate Precipitation: Mechanism of delayed transformation. The Journal of Physical Chemistry B, 124, 6288-6298, https://doi.org/10.1021/acs.jpcd.0c01690.
- Rod, K. A., Smith, A. P., Leng, W., Colby, S., Kukkadapu, R. K., Bowden, M., Qafoku, O., Um, W., Hochella, M. F., Jr., Bailey, V. L. and Renslow, R. S. (2020). Water-dispersible nanocolloids and higher temperatures promote the release of carbon from riparian soil.. Vadose Zone Journal, 19(1), e20077. https://doi.org/10.1002/vzj2.20077
Coming soon...


