X-ray Photoelectron Spectroscopy

X-ray Photoelectron Spectroscopy (XPS) also known as Electron Spectroscopy for Chemical Analysis (ESCA) is a surface analysis technique, used for quantitative analysis of the chemical elements and chemical states from the surface of the material being studied. The average depth of analysis for an XPS measurement is approximately 5 nm. XPS can also be used to obtain depth distribution information by combining XPS measurements with ion milling (sputtering) to characterize thin film structures.
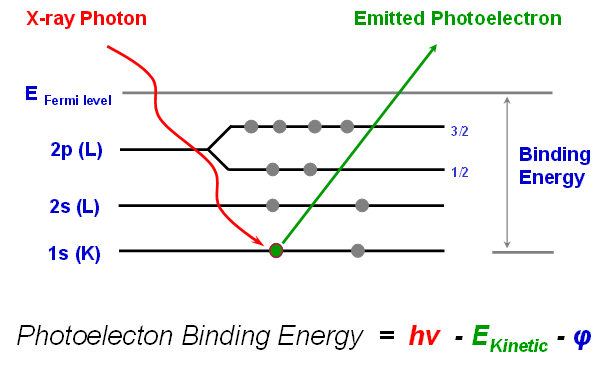
XPS is typically accomplished by exciting a sample surface with mono-energetic Al kα x-rays causing photoelectrons to be emitted from the sample surface. An electron energy analyzer is used to measure the energy of the emitted photoelectrons.
From the binding energy and intensity of a photoelectron peak, the elemental identity, chemical state, and quantity of a detected element can be determined.
APPLICATIONS
- Surface element analysis (except for H and He) of organic and inorganic materials, stains, or residues
- Determining composition and chemical state information from surfaces
- Depth profiling with matrix-level concentrations
- Oxide thickness measurements using angle profile
- Quantitative analysis, including chemical state differences between samples
LIMITATIONS
- Detection limits typically ~ 0.1 atomic %
- Smallest x-ray beam size ~10 µm
- Limited specific organic information
- Can’t detect H and He
- Sample compatibility with ultra high vacuum environment
PHI Quantera SXM
PHI Quantera SXM is a Scanning X-ray Microscopy. It features a focussed, monochromated Al K⍺ x-ray (1486.7 eV) source for small-spot analysis, and it is automated for high sample throughput. Depth profiling can be accomplished with automated ion milling.
TECHNICAL SPECIFICATIONS
- A scanning monochromatic X-ray source with a highly focused beam (~ 9 microns) for rapid chemical state imaging
- 75 mm sample platen, automated sample introduction and stage movement
- Capable of performing analytical surveys, high-resolution multiplexes, sputter depth profiles, line scans, chemical images, automated analyses with user-defined settings
- Hot/Cold stage
Highlight 1 <industry>: Natural Immunogenics Corporation (Nan Qin, PI)
Collaborative Industry Research with Natural Immunogenics Corporation (NIC).
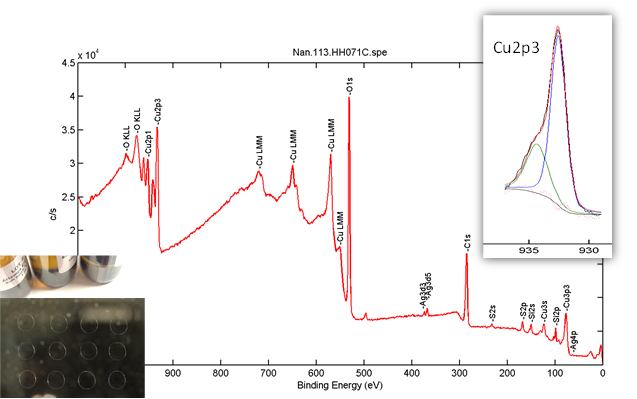
Based in Sarasota, FL, NIC manufactures high-quality silver colloids for health applications. NIC’s colloidal silver is thought to have some of the smallest silver nanoparticles available. The company is also looking beyond silver to develop new products based on copper and gold colloids. As NanoEarth’s first nano/health-related industry user, NIC has already established a lasting partnership with NanoEarth since 2017. This collaborative project aims at a comprehensive materials characterization of NIC’s colloidal products utilizing NanoEarth instruments.
Figure 1. Survey and high resolution (Cu2p) scans on one sample based on a liquid sampling method created by NanoEarth.
Highlight 2 <nanoscale environmental science>: University of Hampton, Hampton, VA (Peter Njoki, PI)
Investigating the Correlation Among Properties, Morphology and Composition of Non-Precious Bimetallic Heterogeneous Nanoparticle Catalysts.
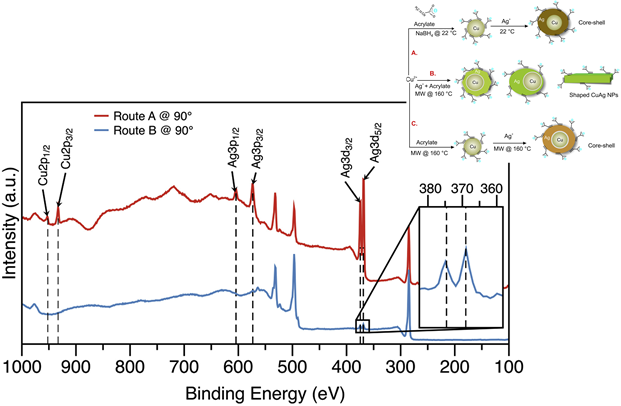
Research in bimetallic NPs has led to intense interest because these materials enable fundamental understanding of the structure-property relationship at the nanoscale, in particular for heterogeneous catalysis associated with energy conversion. This project focuses on synthesis and characterization of shaped copper-silver nanoparticles (CuAg NPs) via microwave irradiation (MW). CuAg NPs were formed by reacting aqueous copper(II) nitrate hemi(pentahydrate) (Cu(NO3)2 •2.5H2O), silver nitrate (AgNO3) and sodium acrylate (C3H3NaO2) at room temperature (RT) and hydrothermal temperature (HT). Shape formation was influenced by the HT and the sequence of reducing the precursors. By reducing Cu(NO3)2 •2.5H2O and AgNO3 with C3H3NaO2 at different temperatures and manipulating the reduction sequence, variable sizes and shapes of CuAg core-shell NPs were formed. When Ag+ ions were reduced on preformed Cu NPs at RT, spherical NPs that featured (111) faucets were produced. The MW synthesis produced octahedra and rods NPs depending on whether Cu2+ and Ag+ ions were co-reduced or Ag+ ions were reduced on preformed Cu NPs.
Figure 2. XPS spectra of Ag 3d, Ag 3p and Cu 2p at tilt angle of 90° for the CuAg NPs synthesized by routes A and B. Inset is the magnified region of Ag 3d3/2 and Ag 3d5/2 for route B synthesis.
Njoki, P. N., Rhoades, A. E. and Barnes, J. I. (2020). Microwave-Assisted synthesis of Anisotropic copper-silver nanoparticles. Materials Chemistry and Physics, 241, 122348. https://doi.org/10.1016/j.matchemphys.2019.122348
Training modules, videos, sample prep, etc.


